Aramid fibre – a modified form of polyamide Nylon
"Aramid" is formed with a combination of words Ar (Aromatic) + amid (Amides)
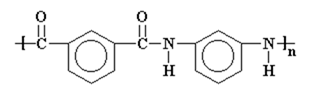
ARAMID is a synthetic fibre that is produced by spinning a solid fibre from solution. The initial preparation of the polymer is generally achieved by a chemical reaction between the amine group and a carboxylic acid halide group.
Aramid fibre characteristics
LONG CHAIN SYNAPTIC POLYMIDE AT LEAST 85% AMIDE (-CO-NH-) linkages are attached directly between two aromatic rings. Molecular structure made of linked Benzene rings and amide bonds.
Fibre structure: The molecules form a planar array with weak interchain hydrogen bonding easily fibrillated up on the fracture. Radially arranged axially pleated crystalline supramolecular sheets.
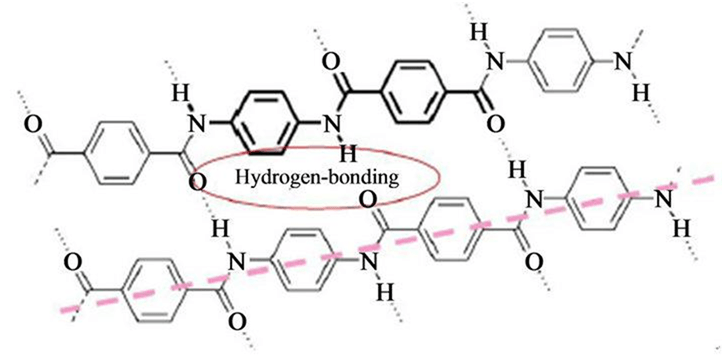
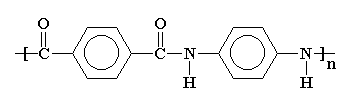
Kevlar is a kind of polyamide. Its amide groups are separated by para-phenylene groups. That is, the amide groups attach to the phenyl rings opposite to each other, at carbons 1 and 4.
Physical properties
-
Optical properties
- Colour—Nomex= white, Kevlar= golden
- Luster—Depends on cross-section shape, circular= more lustre, dog bone=less lustre compared to circular, note—luster can be controlled by finishes.
- Effect of rays—Beta = No effect, Gamma= No effect, UV rays= Starts to degrade and loose strength.
| Manufacturer | Dupont | Hyosung | Kolon | Teijin | Teijin | |
| Trade Name | Kevlar®29 | Alkex®AF-1000 | Heracron®HF200 | Twaron® | Technora® | |
| Specification | UofM | |||||
| Density | (g/cm3) | 1.44 | 1.44 | 1.44 | 1.44 – 1.45 | 1.39 |
| Tenacity | (g/den) | 23 | 23 | 23.0-24.0 | 18.7 – 28.3 | 28.3 |
| Modulus | (Gpa) | 70.33 | 70 – 102 | 8. – 109 | 60 – 120 | 74 |
| Elongation @ Break | (%) | 3.6 | 2.8 – 4.2 | 2.8 – 3.6 | 2.2 – 4.4 | 4.5 |
| Moisture Regain | (%) | 7 | 4.5 | not avail | 3.2 – 5.0 | 1.9 |
| Decomposition | (°C) | 427 – 482 | 500 | not avail | 500 | 500 |
| (°F) | 800 – 900 | 932 | not avail | 932 | 932 | |
| Material | Strength-to-weight KN.m/kg. |
Ultimate Tensile Strength MPa |
Density g/cm3 |
| Kevlar | 2514 | 2757 | 1.44 |
| Carbon Fiber | 2457 | 4137 | 1.75 |
| E Glass Fiber | 1307 | 3450 | 2.57 |
| Carbon Laminate | 785 | 1600 | 1.5 |
| E Glass Laminate | 775 | 1500 | 1.97 |
| Nylon | 69 | 75 | 1.15 |
Carbon Fibre – strong, stiff, & light weight fibres
Properties, process, history, and application of carbon fibres
High Young’s Modulus (structural rigidity): 130-179 GPa. While carbon fiber is 300 Gpa and glass 81 GPa. (Young’s Modulus: Also known as elastic modulus. It defines the relationship between stress and strain in a material)
Properties of Commercial Aramid Fibers
| Material | Young’s Modulus GPa |
| Aramid(such as Kevlar and Twaron) | 70.5-112.4 |
| Nylon | 2-4 |
| Polypropylene | 1.5-2 |
| Fiber Type | Density g/cm3 |
Extension to Break % |
Modulus GPa |
Loop Elongation % |
| Kevlar29 | 1.43 | 3.6 | 70 | 2.1 |
| Kevlar49 | 1.45 | 2.8 | 135 | 1.3 |
| Kevlar119 | 1.44 | 4.4 | 55 | 2.7 |
| Kevlar129 | 1.45 | 3.3 | 99 | |
| Kevlar149 | 1.47 | 1.5 | 143 | 0.6 |
| Nomex | 1.38 | 22 | 17 | |
| Twaron | 1.44 | 3.3 | 79 | |
| Twaron HM | N/a | 2 | 123 | |
| Technora | 1.39 | 4.3 | 70 | |
| Technora V106 | 1.32 | 3.7 | 77 |
Mechanical properties
Nomex and related aramid polymers are related to nylon but have an aromatic backbone, and hence are more rigid and more durable. Nomex is the premier example of a meta variant of the aramids (Kevlar is a para-aramid). Unlike Kevlar, Nomex cannot align during filament formation and has poorer strength. However, it has excellent thermal, chemical, and radiation resistance for polymer materials.
A series of synthetic polymers in which repeating units containing large phenyl rings are linked together by amide groups. Amide groups (CO-NH) form strong bonds that are resistant to solvents and heat. Phenyl rings (or aromatic rings) are bulky six-sided groups of carbon and hydrogen atoms that prevent polymer chains from rotating and twisting around their chemical bonds.
- Moisture regains: Kevlar—1.2—4.3%, Nomex—6.5%, Non -flexible chains. Reason: two alternate rings try to oppose each other by repulsion of aromatic rings which keeps the fibres straight and non-flexible. Hydrogen bonding between chains thereby forming a sheet-like structure (OF FIBRILS). Fibrils (sheets)separated by elongated voids. Reason: because each sheet is a plane whose both faces are negatively charged because of Aromatic rings due to this elongated void is created between fibril sheets.
- Fibre properties: They are characterized by medium to ultra-high strength, medium to low elongation and moderately high to ultra-high modulus with the densities ranging from 1.38g/cm3 to 1.47g/cm3. Heat-resistant and flame-resistant aramid fibres contain high proportion or meta-oriented phenylene rings, whereas ultra-high strength high-modulus fibres contain mainly para-oriented phenylene rings.
- Aramid yarn has a breaking tenacity of 3045 MPa, in other words, more than 5 times than this of steel (under water, aramid is 4 times stronger) and twice than this of glass fibre or nylon. High strength is a result of its aromatic and amide group and high crystallinity. Aramid retains strength and modulus at temperatures as high as 300 degrees Celsius. It behaves elastically under tension. When it comes to severe bending, it shows non-linear plastic deformation. With tension fatigue, no failure is observed even at impressively high loads and cycle times. Creep strain for aramid is only 0.3%.
- Chemical properties: All aramids contain amide links that are hydrophilic. However, not all aramid products absorb moisture the same. The PPD-T (poly-phenylene terephthalamide) fibre has very good resistance to many organic solvents and salt, but strong acids can cause substantial loss of strength. Aramid fibres are difficult to dye due to their high Tg. Also, the aromatic nature of para-aramid is responsible for oxidative reactions when exposed to UV light, that leads to a change in colour and loss of some strength.
- Resistance to Alkalis—Dilute 9AT ROOM TEMP.) —-NO EFFECT TO Nomex, and Kevlar but at high temperatures, it weakens both. Resistance to Dye—Because of high negative charge repulsion it is hard to dye but in extreme conditions support electrophilic substitution reactions. Resistance to bleach—Resistance to bleach that is why it is hard to change its natural golden colour of Kevlar.
- Thermal properties: Aramid fibres do not melt in the conventional sense but decompose simultaneously. They burn only with difficulty because of Limited Oxygen Index (LOI) values. It should be mentioned that at 300 degrees Celsius some aramid types can still retain about 50% of their strength. Aramids show high crystallinity which results in negligible shrinkage at high temperature.
To sum up, aramid general characteristics are:
- High strength
- Resistance to absorption
- Resistance to an organic solvent, good chemical resistance
- No conductivity
- No melting point
- Low flammability
- Excellent heat, and cut resistance
- Sensitive to acids and ultraviolet radiation